PrismCore platform and
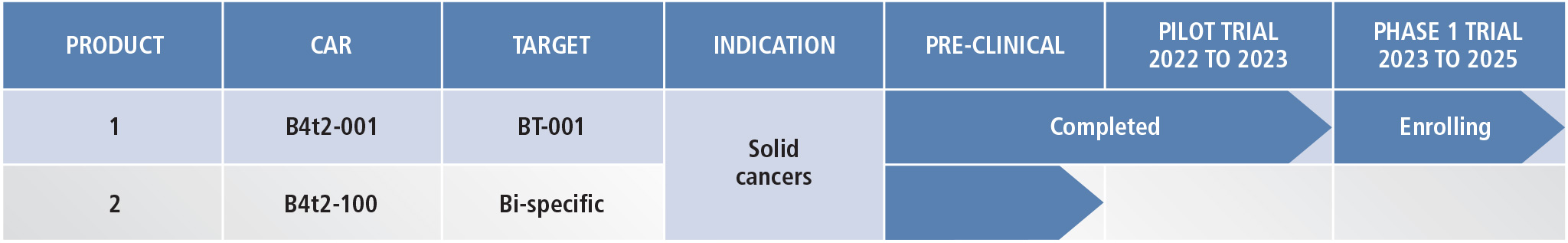
Bio-Engine fuel the pipeline for production of autologous CAR-T to target solid tumors. Bio4t2's first product candidate B4t2-001, is undergoing evaluation in a series of phase 1
clinical trials. This CAR-T targets a unique and high value antigen BT-001, that is commonly present at elevated levels on the surface of multiple invasive cancers, including colorectal, gastric, pancreatic and lung cancers. The data from the first-in-human pilot study (using the investigator-initiated trial mechanism,
ClinicalTrials.gov ID NCT05621486) in People's Republic of China yielded encouraging results including potent engraftment of CAR-T, even without preparative chemotherapy (lymphodepletion) and anti-tumor effects. The trial has been expanded to take advantage of these learnings to further understand the pharmacokinetics and pharmacodynamics of infused T cells given without preparative chemotherapy, to administer the product by intravenous and intraperitoneal routes, and to deliver multiple rounds of CAR-T.
Bio4t2 has also established the technology and methods of designing and developing bi-specific CARs with added specificity for two cell surface antigens. Our autologous
Bi-CAR-T emerging from the PrismCore and Bio-Engine technologies will soon enter clinical trials as our second product candidate.